Drug development
Program transferred from the US
Obstructive Sleep Apnoea (OSA)
Initial use case obstructive sleep apnoea followed by anorexia
Investment Highlights
- Australian Contract Research Organization contracted
- Australian manufacturer contracted
- Term sheet from source of debt funding to finance 80% of R&D tax credit
- Plans to become listed for public trading
- Auditor engaged
- Accountants engaged
- Lawyers engaged
- First equity finance term sheet in place on same terms as offering for US$3.125M (~AUD4.687M)
De-risked drug commercialisation for millions of sleep apnoea sufferers
ResolutionRx Ltd is focused on developing and repurposing an FDA-approved drug Dronabinol in helping Obstructive Sleep Apnoea (“OSA”) sufferers to better manage their condition.
Dronabinol is a cannabinoid drug with FDA approval for the treatment of AIDS-related anorexia and chemotherapy-induced nausea and vomiting.
Two statistically significant Phase 2 clinical trials completed. New improved formulation created in preparation for Phase 3. This repurposing strategy should only require approval by the U.S. FDA of a 505(b)(2) new drug application (“NDA”), an efficient regulatory pathway to further approval for OSA treatment that allows the use of publicly available data. Similarly efficient regulatory pathways are available elsewhere in the world.
Dronabinol, a synthetic version of ∆-9-THC, a naturally occurring substance in the cannabis plant, has already demonstrated significant improvement in the symptoms of OSA in two Phase 2 clinical trials in the United States.
A serious respiratory disorder that affects millions of people globally
OSA is a serious respiratory disorder that impacts more than 90 million in Australia, the United States, Germany and the United Kingdom. There are an estimated 1 billion cases worldwide
Corporate Structure
ResolutionRx Ltd was formed in Australia in January 2023 by RespireRx as an unlisted public company. RespireRx Pharmaceuticals Inc. (US OTC: RSPI) has contributed via sublicense and license with ResolutionRx, its cannabinoid drug development program subject to certain liabilities.
ResolutionRx is engaging in the R&D associated with that program, initially for the development of a new formulation of dronabinol for use in Phase 3 clinical trial and the filing of regulatory approval for the treatment of obstructive sleep apnoea (“OSA”).
The current total budget for that program over the next several years is approximately A$24.8M, most, but not all of which is expected to be eligible for the R&D tax refund expected to be 43.5%.

Raising an additional A$18M (to achieve A$22.7M of which A$4.7M is the subject of an existing term sheet with a single investor) on a pre-money valuation of A$33.75M by way of series A preference shares that will convert into ordinary shares.
Funds will be used for Research and Development and General and Administrative Purposes.


The content on this site is provided solely for information purposes, is not a recommendation or an offer to buy or sell a security and is not warranted to be correct, complete or accurate. This site is intended for Accredited/Sophisticated/Wholesale investors only. By continuing with an investment, you are self-accrediting that you so qualify. To the extent permitted by law, neither PrimaryMarkets, its affiliates, nor the content providers (such as the issuers of securities who appear on the site) are responsible for any investment decisions, damages or losses resulting from, or related to, the content, data and analyses or their use. The investment opportunities on this site and any statements made about them by their issuers are not vetted or verified by PrimaryMarkets. Investing in securities in private or unlisted companies and Funds is speculative and involves a high degree of risk. The investor must be prepared to withstand a total loss of their investment. We strongly encourage the investor to seek independent financial, professional or investment advice before investing in securities. The presence of an investment opportunities on this site should not be interpreted as an implied endorsement of it by PrimaryMarkets. Some content provided may constitute a summary or extract of another document. Past performance does not necessarily indicate future performance. The content on this site was current as at date of initial publication but may not be current as at the date of your viewing. For a more complete understanding of all the terms and conditions of your use of this site click here.
ResolutionRx, an unlisted public Australian company, is focused on repurposing, developing and commercialising proprietary pharmaceutical product candidates, our first candidate being the repurposing of a pharmaceutical cannabinoid.
ResolutionRx was created by RespireRx Pharmaceuticals Inc. (US OTC: RSPI) (RespireRx), which has contributed its cannabinoid drug development program to ResolutionRx.
ResolutionRx is continuing the research and development (R&D) associated with that program, initially for the development of a new formulation of dronabinol for use as a treatment for obstructive sleep apnoea (“OSA”).
Team Management
Arnold Lippa - Acting Co-CEO
Jeff E Margolis - Acting Co-CEO and Chief Financial Officer
Team Directors
Arnold Lippa - Executive Chairman
Jeff E Margolis - Member of the Board
Michael Burfield - Member of the Board
Ty Burton - Member of the Board
Click here to view our latest News and Media.
• By clicking the enquire button on the card view
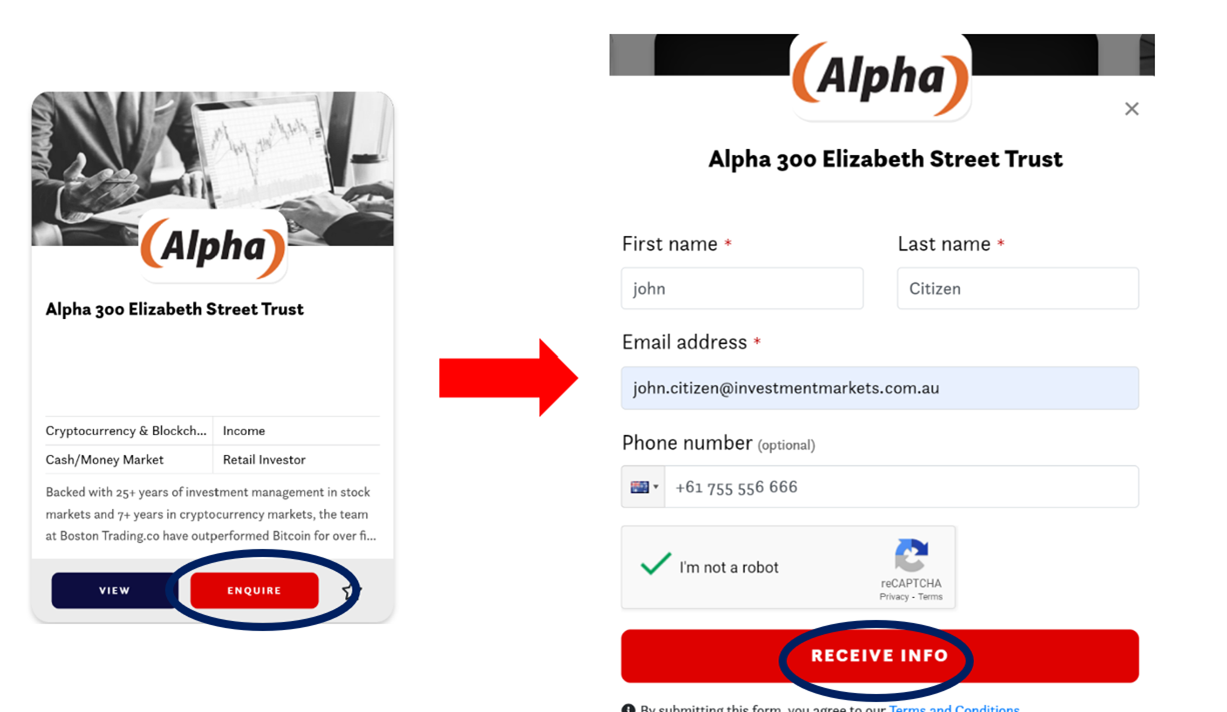
Or
• By selecting “Receive Info” when viewing the listing
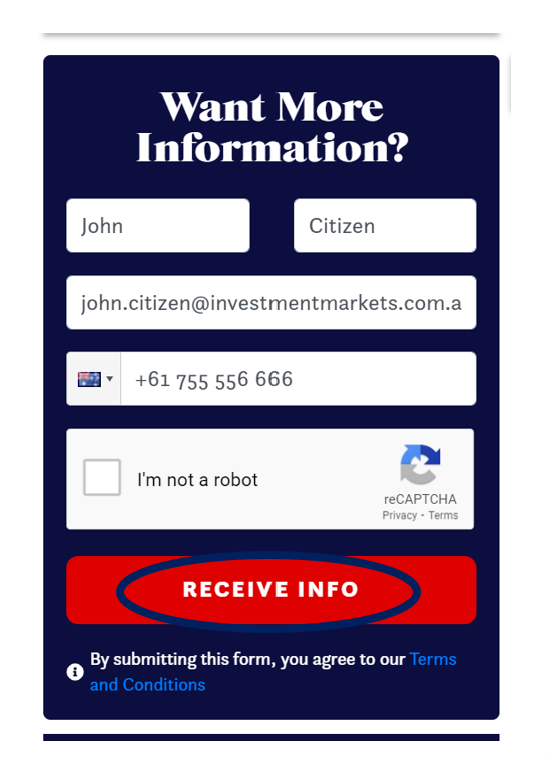
We will respond using your preferred communication method (i.e., email or phone).
Tags
Published by PrimaryMarkets Pty Ltd

PRIVATE ASSETSINVESTOR EDUCATION

PRIVATE ASSETSINVESTOR EDUCATION

SHARESINVESTOR EDUCATION
Published by PrimaryMarkets Pty Ltd
Statutory Statement
This offer of scheme interests is available to wholesale clients only. This product listing was vetted by and approved by the product issuer identified above before publishing. Investment Markets (Aust) Pty Ltd AFSL 527875 (IM) is not the issuer of the product.
General Disclaimer
IMPORTANT STATEMENT ABOUT YOUR USE OF THIS SITE
Information on this site is intended for Australian users only.
This site is operated by Investment Markets (Aust) Pty Ltd. (ACN 634 057 248) (IMA, we, us and our), the holder of Australian Financial Services Licence (AFSL) no. 527875. The content is provided solely for information purposes, is not a recommendation or an offer to buy or sell a security, and is not warranted to be correct, complete or accurate. To the extent permitted by law, neither IMA, its affiliates, nor the content providers (such as the issuers of securities who appear on the site) are responsible for any investment decisions, damages or losses resulting from, or related to, the content, data and analyses or their use. The investment products on this site and any statements made about them by their issuers are not vetted, verified or researched by IMA. The presence of an investment product on this site should not be interpreted as an implied endorsement of it by IMA. Certain content provided may constitute a summary or extract of another document such as a Product Disclosure Statement. To the extent any content is general advice, it has been prepared by IMA. Any general advice has been provided without reference to your investment objectives, financial situations or needs. For more information refer to our Financial Services Guide. To obtain advice tailored to your situation, contact a financial advisor. You should consider the advice in light of these matters and, if applicable, the relevant Product Disclosure Statement (or other offer document) before making any decision to invest. Past performance does not necessarily indicate an investment product’s future performance. The content is current as at date of initial publication and may not be current as at your date of viewing. For a more complete understanding of all the terms and conditions of your use of this site click here.


